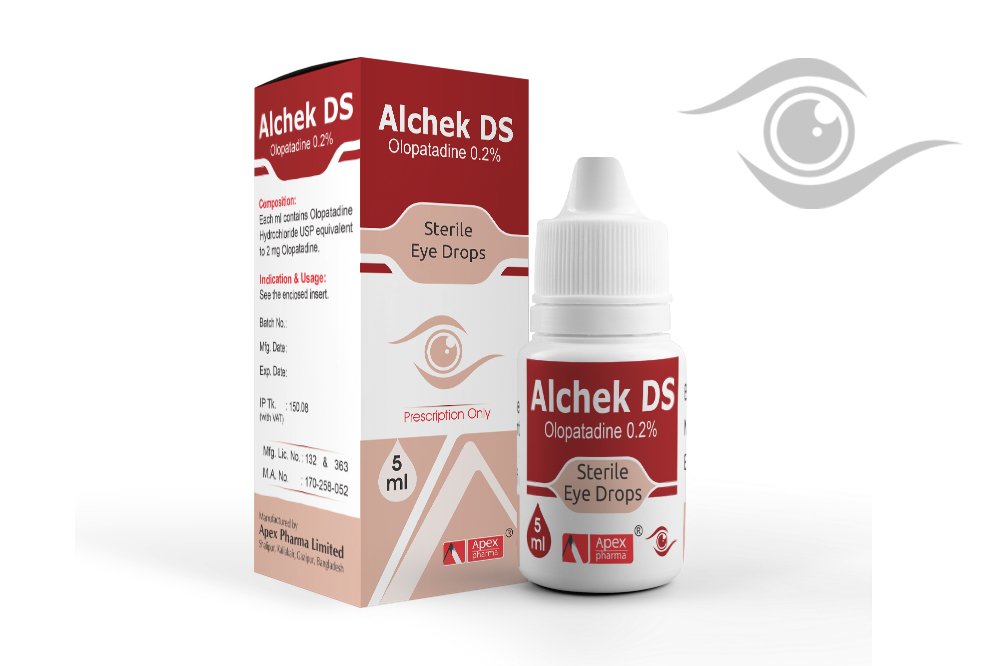
Alchek DS
Home / By Brand Name / Alchek DS
Composition
Alchek: Each ml contains Olopatadine Hydrochloride USP equivalent to 1 mg Olopatadine.
Alchek DS: Each ml contains Olopatadine Hydrochloride USP equivalent to 2 mg Olopatadine.
Pharmacology
Olopatadine is a sterile ophthalmic solution containing Olopatadine, a relatively selective H1 -receptor
antagonist and inhibitor of histamine release from the mast cell for topical administration to the eyes.
Olopatadine is an inhibitor of the release of histamine from the mast cell and a relatively selective histamine
H1–antagonist that inhibits the in vivo and in vitro type -1 immediate hypersensitivity reaction including inhibition
of histamine induced effects on human conjunctival epithelial cells. Olopatadine is devoid of effects on
alpha-adrenergic, dopamine and muscarinic type -1 and 2 receptors.
Indication
Olopatadine is indicated for the treatment of the signs and symptoms of allergic conjunctivitis.
Dosage & Administration
Alchek: The recommended dose is one drop in each affected eye two times per day at an interval of 6 to 8
hours.
Alchek DS: The recommended dose is one drop in each affected eye once per day.
Contraindication
It is contraindicated in patients with a known hypersensitivity to Olopatadine Hydrochloride or any components
of Olopatadine Eye drop.
Precaution
To prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or
surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use. Patients should
be advised not to wear a contact lens if their eye is red. It should not be used to treat contact lens related
irritation. Benzalkonium chloride, the preservative used in Olopatadine eye drops may be absorbed by soft
contact lenses. Patients who wear soft contact lenses and whose eyes are not red should be instructed to wait
at least ten minutes after instilling Olopatadine before they insert their contact lenses.
Side-effect
Headaches have been reported at an incidence of 7%. The following adverse experiences have been reported
in less than 5% of patients: asthenia, blurred vision, burning or stinging, cold syndrome, dry eye, foreign body
sensation, hyperemia, hypersensitivity, keratitis, lid edema, nausea, pharyngitis, pruritus, rhinitis, sinusitis, and
taste perversion. Some of these events were similar to the underlying disease being studied.
Overdose
No information available.
Drug Interaction
No information provided.
Store in cool, dry place and away from light. Keep out of the reach of children. Use within one month after the
first opening.
PRODUCTS