Montelon
Home / By Brand Name / Montelon
Composition
Montelon Kidz Tablet: Each chewable tablet contains Montelukast 4 mg as Montelukast Sodium USP.
Montelon 5 Tablet: Each chewable tablet contains Montelukast 5 mg as Montelukast Sodium USP.
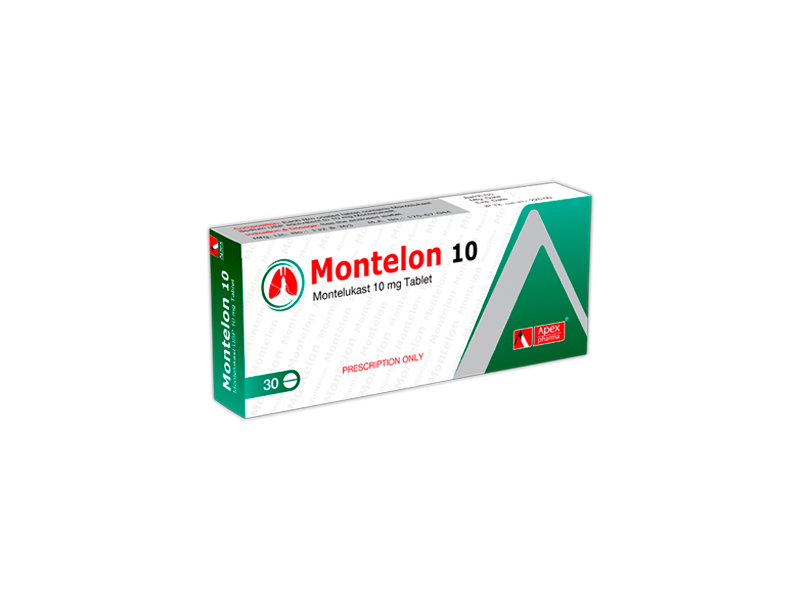
Montelon 10 Tablet: Each film coated tablet contains Montelukast 10 mg as Montelukast Sodium USP.
Pharmacology
Montelukast is a selective and orally active leukotriene receptor antagonist that specifically inhibits cysteinyl
leukotriene receptor.
Indication
Montelukast is indicated for the prophylaxis and chronic treatment of asthma. It is also indicated for the relief of
seasonal allergic rhinitis in adults and pediatric patients and exercise induced bronchoconstriction.
Dosage and Administration
Prophylaxis of Asthma:
Adults and Children (over 15 years): 10 mg once daily in the evening.
Children (6 years-15 years): 5 mg once daily in the evening.
Children (6 months-6 years): 4 mg once daily in the evening.
Seasonal Allergic Rhinitis:
Adults and Children (over 15 years): 10 mg once daily in the evening.
Exercise Induced Bronchoconstriction:
10 mg tablet at least 2 hours before exercise.
Contraindication
Hypersensitivity to Montelukast or any component of this product.
Precaution
Do not prescribe Montelukast to treat an acute asthma attack. Patients with known aspirin sensitivity should
continue to avoid aspirin or NSAID agents while taking Montelukast.
Side effect
Montelukast appears to be well tolerated. The most common adverse effects reported are headache, rash,
dyspepsia, dizziness, edema, tremor, suicidal thoughts and abdominal pain.
Use in special group
Pregnancy: Montelukast has been assigned to pregnancy category B. It should be used during pregnancy only
if clearly needed.
Lactation: It is not known if Montelukast is excreted in human milk.
Pediatric use: Montelukast has been studied in pediatric patients (2 to 14 years of age). Studies have shown
that Montelukast does not affect the growth rate of pediatric patients.
Drug Interaction
Montelukast may be administered with therapies routinely used in the prophylaxis and chronic treatment of
asthma. In drug interaction studies, the recommended clinical dose of Montelukast does not have clinically
important effects on the following medicinal products: Theophylline, Prednisone, Prednisolone, Oral
Contraceptives, Terfenadine, Digoxin and Warfarin. Caution should be exercised, particularly in children, when
Montelukast is co-administered with Phenytoin, Phenobarbital and Rifampicin.
Overdose
No specific information is available on the treatment of overdose with Montelukast.
Keep away from light & moisture and store below 30o C. Keep out of the reach of children.
PRODUCTS