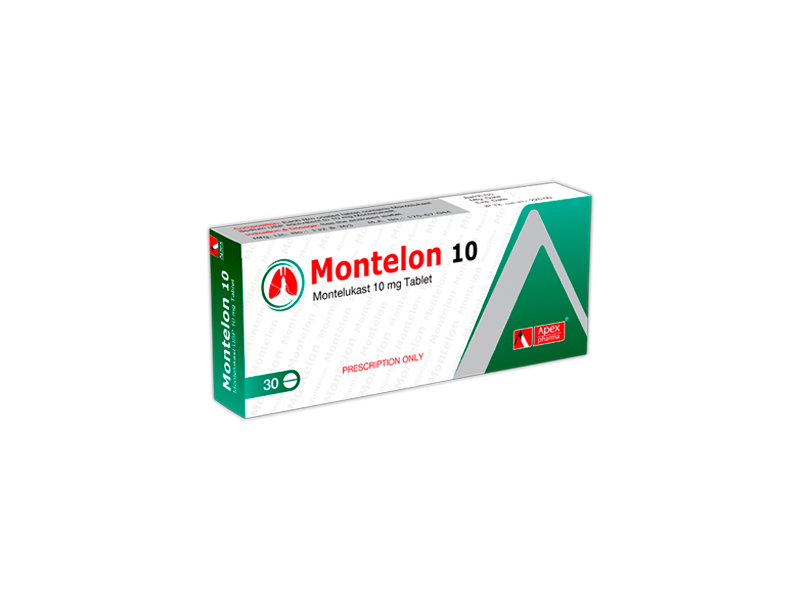
Napronex
Home / By Brand Name / Napronex
COMPOSITION:
Napronex 375/20 Tablet: Each delayed release tablet contains 375 mg of Naproxen USP and 20 mg of
Esomeprazole as Esomeprazole Magnesium Trihydrate BP
Napronex 500/20 Tablet: Each delayed release tablet contains 500 mg of Naproxen USP and 20 mg of
Esomeprazole as Esomeprazole Magnesium Trihydrate BP
DESCRIPTION:
Napronex consists of an immediate release Esomeprazole magnesium layer and an enteric coated Naproxen
core. As a result, Esomeprazole is released first into the stomach, prior to the dissolution of Naproxen in the
small intestine. Naproxen is a NSAID with analgesic and antipyretic properties. The mechanism of action of
Naproxen is to inhibition of the prostaglandin synthesis. Esomeprazole is a proton pump inhibitor that suppresses
gastric acid secretion by specific inhibition of the H+ /K+ -ATPase in the gastric parietal cell. By acting specifically
on the proton pump, Esomeprazole blocks the final step in acid production, thus reducing gastric acidity.
INDICATIONS:
It is indicated for the relief of signs and symptoms of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis,
dysmenorrhea and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID
associated gastric ulcers.
DOSAGE & ADMINISTRATION:
One Napronex 375/20 mg or Napronex 500/20 mg tablet twice daily.
The tablets are to be swallowed whole with liquid. Do not split, chew, crush or dissolve the tablet. The tablet is to
be taken at least 30 minutes before meals.
Elderly patients: Studies indicate that although total plasma concentration of Naproxen is unchanged, the
unbound plasma fraction of Naproxen is increased in the elderly. Use caution when high doses are required and
some adjustment of dosage may be required in elderly patients. As with other drugs used in the elderly use the
lowest effective dose.
Patients with renal impairment: Naproxen containing products are not recommended for use in patients with
moderate to severe renal impairment (creatinine clearance<30 mL/min).
Hepatic insufficiency: Not recommended in patients with severe hepatic impairment because Esomeprazole
dose should not exceed 20 mg daily in these patients.
Children: Use in children less than 18 years has not been established yet.
SIDE EFFECTS:
In general, Napronex is well tolerated. The most common adverse reactions in clinical trials (>5%): erosive
gastritis, dyspepsia, gastritis, diarrhea, gastric ulcer, upper abdominal pain, nausea etc.
PRECAUTIONS:
Patients with known CV disease/risk factors may be at greater risk. Napronex should be used with caution in
patients with fluid retention or heart failure.
CONTRAINDICATIONS:
• Known hypersensitivity to any component of Napronex or substituted benzimidazoles
• History of asthma, urticaria or other allergic-type reactions after taking aspirin or other NSAIDs
• Use during the peri-operative period in the setting of coronary artery bypass graft (CABG)
surgery
• Late pregnancy
USE IN PREGNANCY & LACTATION:
In pregnancy: Pregnancy category C. In late pregnancy, it should be avoided because it may cause birth defects.
In lactation: Napronex should not be used in nursing mothers due to the naproxen component.
DRUG INTERACTION:
• Concomitant use of NSAIDs may reduce the antihypertensive effect of ACE Inhibitors,
diuretics, and beta-blockers
• Concomitant use of Napronex and warfarin may result in increased risk of bleeding
complications.
• Esomeprazole inhibits gastric acid secretion and may interfere with the absorption of drugs
where gastric pH is an important determinant of bioavailability (eg, ketoconazole, iron salts
and digoxin).
OVERDOSE:
There is no clinical data on overdosage with Napronex.
Overdose of Naproxen: Significant naproxen overdosage may be characterized by lethargy, drowsiness,
epigastric pain, abdominal discomfort, heartburn, indigestion, nausea, transient alterations in liver function,
hypoprothrombinemia, renal dysfunction, metabolic acidosis, apnea, vomiting etc.
Overdose of Esomeprazole: The major signs of acute toxicity were reduced motor activity, changes in respiratory
frequency, tremor and intermittent clonic convulsions etc.
Store in cool, dry place & away from light. Keep out of the reach of children.
PRODUCTS