Texit
Home / By Brand Name / Texit
Composition
Texit-200 Capsule: Each capsule contains 200 mg of Cefixime as Cefixime Trihydrate USP.
Texit-400 Capsule: Each capsule contains 400 mg of Cefixime as Cefixime Trihydrate USP.
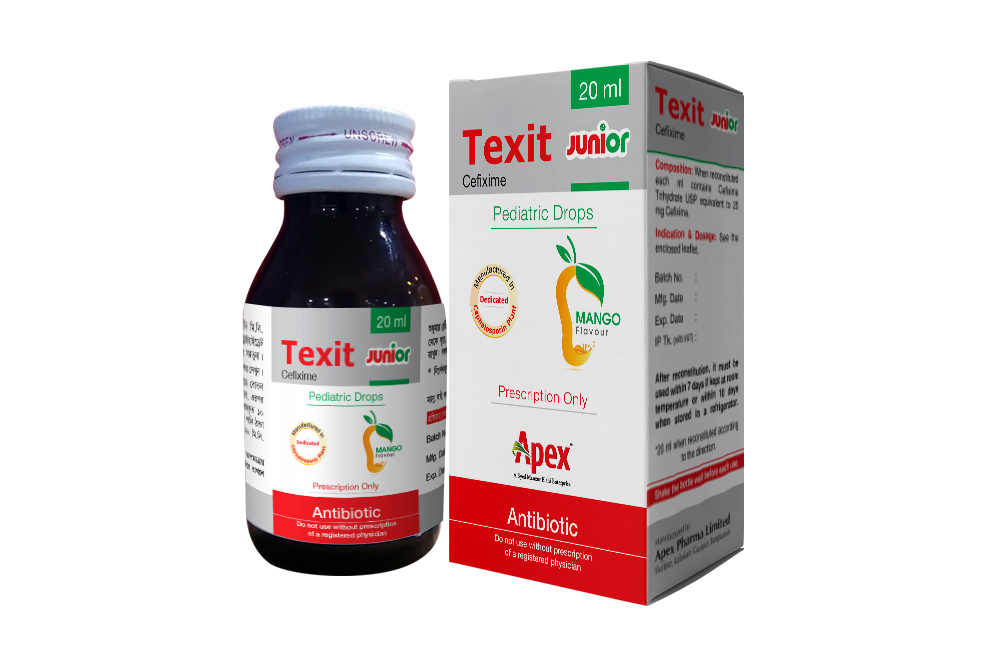
Texit Junior Pediatric Drops: When reconstituted each ml contains Cefixime Trihydrate USP equivalent to 25 mg Cefixime.
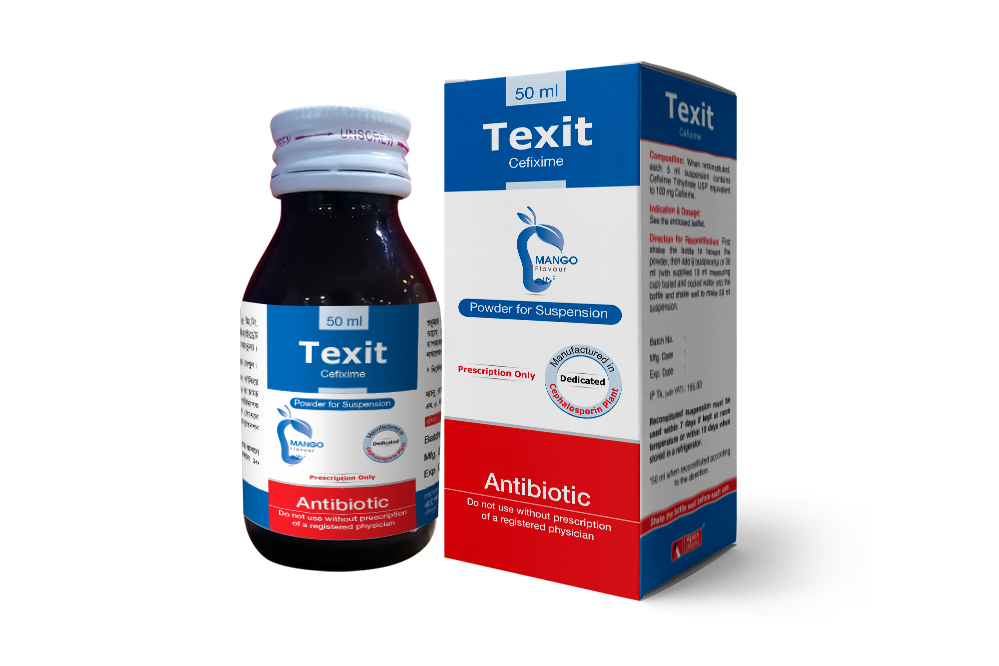
Texit 50 ml PFS: When reconstituted each 5 ml suspension contains Cefixime Trihydrate USP equivalent to 100 mg Cefixime.
Texit Large PFS: When reconstituted each 5 ml suspension contains Cefixime Trihydrate USP equivalent to 100 mg Cefixime.
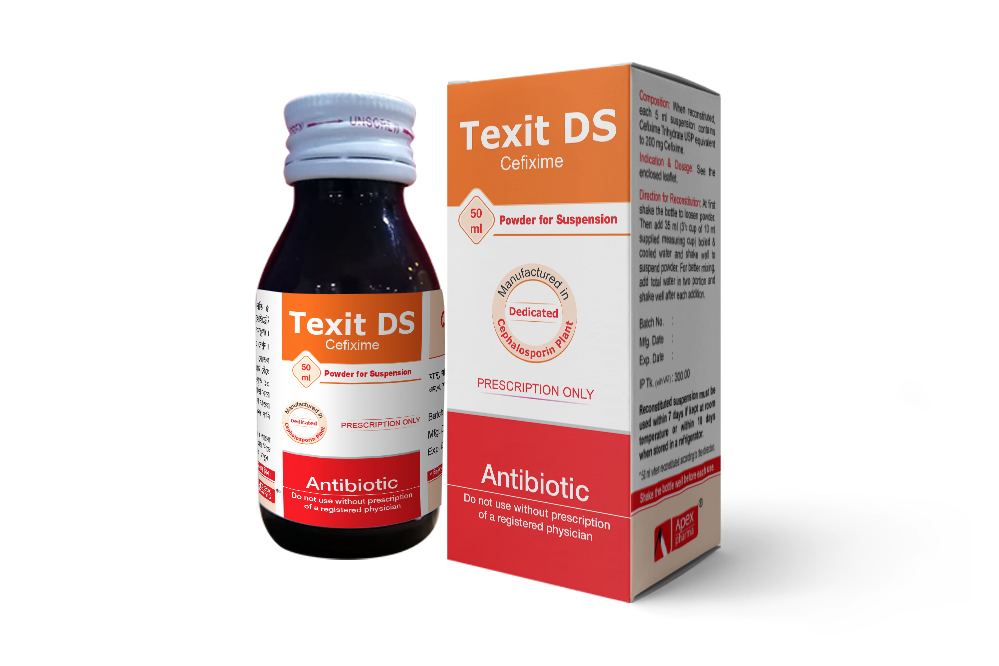
Texit DS PFS: When reconstituted each 5 ml suspension contains Cefixime Trihydrate USP equivalent to 200 mg Cefixime.
Pharmacology
Texit (Cefixime) is a broad spectrum antibiotic of 3rd generation Cephalosporin group. Cefixime has a longer duration of action than the other Cephalosporins that are active by mouth. It is a bactericidal antibiotic and is stable to many beta-lactamases. Cefixime kills bacteria by interfering in the synthesis of the bacterial cell wall. Peak plasma concentration attained within 2-6 hrs, plasma half-life usually 3-4 hrs and about 65% is bound to plasma protein.
Indication
Texit (Cefixime) is indicated for the treatment of following infections:
- Upper and lower respiratory tract infections: acute bronchitis and acute
exacerbation of chronic bronchitis, acute sinusitis, pneumonia
caused by Streptococcus pneumoniae and Haemophilus influenzae. - Otitis media caused by Haemophilus influenzae, Moraxella catarrhalis & S. pyogenes
- Pharyngitis & Tonsillitis caused by S. pyogenes
- Uncomplicated urinary tract infections caused by Escherichia coli & Proteus mirabilis
- Uncomplicated cervical and rectal infections
- Uncomplicated gonorrhea (Cervical & Urethral) caused by Neisseria gonorrhea
- Multi-Drug Resistant (MDR) enteric fever
Storage ConditionsDosage and Administration
Community Acquired Pneumonia (CAP): Children (6 months to 12 years of age): 8 mg/kg once daily or 4 mg/kg every 12 hours for 10-14
days. Adult: 400 mg once daily or 200 mg every 12 hours for 10-14 days
Acute Otitis Media (AOM): Children (6 months to 12 years of age): 8 mg/kg once daily or 4 mg/kg every 12 hours for 10-14 days Adult:
400 mg once daily or 200 mg every 12 hours for 10-14 days
Pharyngitis and Tonsillitis: Children (6 months to 12 years of age): 8 mg/kg once daily or 4 mg/kg every 12 hours for 10-14 days Adult:
400 mg once daily or 200 mg every 12 hours for 10 days
Acute Exacerbations of Chronic Bronchitis (AECB): Children (6 months to 12 years of age): 8 mg/kg once daily or 4 mg/kg every 12
hours for 10-14 days Adult: 400 mg once daily or 200 mg every 12 hours for 10-14 days
Acute Bacterial Sinusitis: 8 mg/kg once daily or 4 mg/kg every 12 hours for 10-14 days.
Uncomplicated Urethral, Endocervical or Rectal Gonorrhea: Children weighing < 45 kg: 8 mg/kg (upto 400 mg) as single dose
Typhoid Fever: 20 mg/kg once daily or 10 mg/kg every 12 hours for 14 days.
Children beyond neonatal period: 8 mg/kg daily in 1 or 2 equally divided doses for treatment of mild or moderate infections.
In renal impairment: Dose should be adjusted according to Creatinine Clearance (CC). CC 20-60 ml/min: 75% of standard dose and
CC<20 ml/min: 50% of standard dose.
Contraindication
Texit (Cefixime) is contraindicated in known hypersensitivity to Cefixime or other antibiotics of Cephalosporin group.
Precaution
Texit (Cefixime) should be administered with caution in renal impairment, a history of colitis and on anticoagulant therapy. Like other antibiotics, pseudomembranous colitis has been reported with Cefixime.
Side Effect
Cefixime is generally well tolerated. The majority of adverse reactions observed in clinical trials were mild and self-limiting in nature. Gastrointestinal disturbances: Diarrhea, abdominal pain, dyspepsia, nausea and vomiting. Hypersensitivity Reactions: Skin rashes, urticaria, pruritus, angioedema and facial edema. Hepatic: Hepatitis, jaundice. Renal: Transient elevations in BUN or creatinine, renal failure. Central Nervous System: Headache, dizziness & seizures. Hemic and Lymphatic Systems: Thrombocytopenia, leukopenia, neutropenia and eosinophilia. Prolongation in prothrombin time was seen rarely. Other: Genital pruritus, vaginitis, candidiasis, toxic epidermal necrolysis.
Pregnancy and Lactation
Pregnancy: Pregnancy category B. Although the use of Cefixime in pregnancy not known to be harmful, this drug should be used during pregnancy only if clearly needed. Lactation: Since Cefixime is excreted in breast milk in low concentration, caution should be exercised when Cefixime is administered to a nursing woman.
Drug Interaction
Carbamazepine: Elevates Carbamazepine blood level. Warfarin: Increased prothrombin-time.
Direction for Reconstitution of Texit Suspension:
Shake the bottle until all powder flows freely. Then add boiled & cool water 12.5 ml for Texit Junior Pediatric Drops, 30 ml (3 cup of 10 ml supplied measuring cup) for 50 ml Texit PFS, 60 ml (6 cup of 10 ml supplied measuring cup) for Texit Large PFS and 35 ml (31/2 cup of 10 ml supplied measuring cup) for Texit DS PFS shake well to mix powder. For better mixing add total water in two portions and shake well each time.
Note: After reconstitution keep the bottle tightly closed and shake the bottle well before each use. Reconstituted suspension must be used within 10 days if kept at room temperature or within 14 days when stored in a refrigerator. For 50 ml Texit PFS and Texit Junior pediatric drops, reconstituted suspension must be used within 7 days if kept at room temperature or within 10 days when stored in a
refrigerator.
Over Dose
Gastric lavage may be indicated; otherwise, no specific antidote exists. Cefixime is not removed in significant quantities from the circulation by hemodialysis or peritoneal dialysis. Adverse reactions in small numbers of healthy adult volunteers receiving single doses upto 2 g of cefixime didnot differ from the profile seen in patients treated at the recommended doses.
Keep away from light & moisture and store below 30o C. Keep out of the reach of children
PRODUCTS